Imagine your cells as bustling cities under siege; oxidative stress is the relentless bombardment they face daily, and NRF2 acts as the city's defense mechanism.
You've likely heard that oxidative stress plays a pivotal role in cancer development, but the intricate dance between this physiological stress and NRF2 is less understood. NRF2, a transcription factor, orchestrates the cellular response to oxidative stress, influencing cancer progression and resistance.
Its dual role as a protector and potential facilitator of cancerous growth underscores the complexity of targeting NRF2 in cancer therapy. Unpacking the nuances of this relationship offers insights into novel therapeutic strategies, yet challenges in harnessing NRF2's full potential persist.
The question then arises: how can we navigate these complexities to benefit cancer treatment?
NRF2 Explained
Nuclear factor erythroid 2-related factor 2 (NRF2) plays a critical role in cellular defense mechanisms against oxidative stress by regulating antioxidant response element (ARE)-driven gene expression. You must understand that NRF2's activation and subsequent transcriptional activity are pivotal in maintaining cellular homeostasis. Its ability to upregulate genes involved in detoxification and antioxidant processes showcases its significance in protecting cells from oxidative damage.
Diving deeper, you'll find that the regulation of NRF2 is intricately designed. Under basal conditions, NRF2 is kept in check by KEAP1, a cytoplasmic inhibitor that targets NRF2 for ubiquitination and proteasomal degradation. However, upon oxidative stress, NRF2 escapes KEAP1's hold, translocates to the nucleus, and binds to ARE, thus initiating the transcription of its target genes.
This regulatory mechanism underscores NRF2's adaptability to oxidative challenges, reinforcing its role as a master regulator of antioxidant responses. Research evidences that dysregulation of NRF2 pathway can significantly impact cellular resilience against oxidative stress, further implicating its involvement in various diseases, including cancer. Hence, understanding NRF2's regulation and function provides a critical insight into its potential as a therapeutic target in oxidative stress-related conditions.
Oxidative Stress Basics
To understand the body's battle against cellular damage, it's essential to grasp the fundamentals of oxidative stress, a condition where an imbalance between free radicals and antioxidants leads to tissue harm and plays a crucial role in disease progression, including cancer.
Free radicals are highly reactive molecules with unpaired electrons, seeking stability through electron pairing. This quest causes damage to proteins, lipids, and DNA, potentially leading to cellular dysfunction and death.
Antioxidants, on the other hand, neutralize free radicals by donating electrons without becoming destabilized themselves, thus preventing potential cellular damage. However, when the production of free radicals overwhelms the body's antioxidant defenses, oxidative stress occurs. This imbalance isn't merely a consequence of external factors like pollution or UV exposure but also arises from normal cellular processes, such as mitochondrial respiration, which inadvertently generates free radicals.
The body's inability to counteract or repair the damage caused by oxidative stress can lead to chronic inflammation, cellular aging, and the activation of oncogenes, which are genes that can lead to cancer when mutated or expressed at high levels. Understanding this delicate balance between oxidative stress and antioxidant defense mechanisms is fundamental in deciphering the complex relationship between oxidative stress and cancer development, excluding the direct role of NRF2 in this process.
NRF2 in Cancer Development
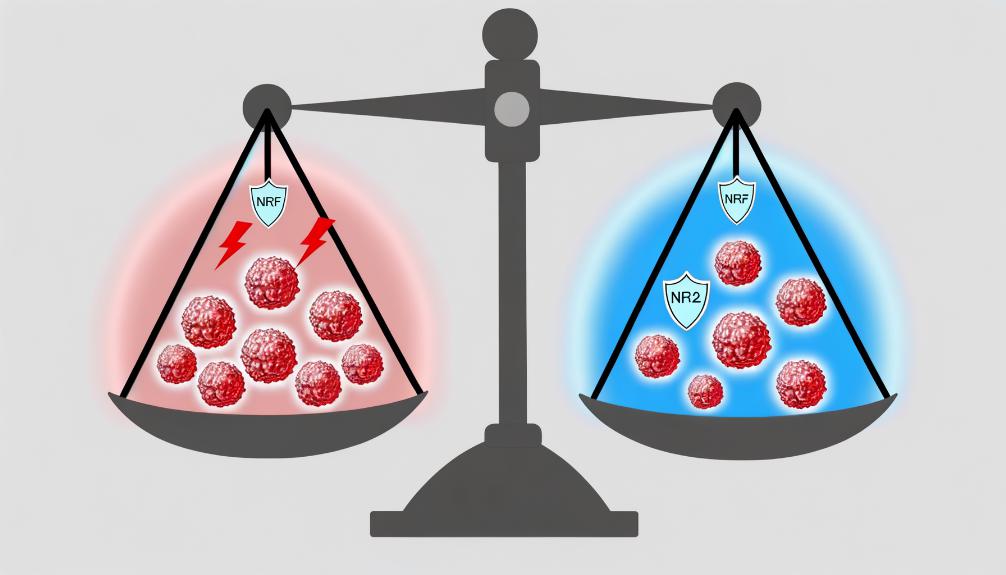
Understanding the balance between oxidative stress and antioxidant defenses sets the stage for exploring how NRF2, a critical regulatory protein, influences cancer development by modulating cellular responses to oxidative damage. NRF2's role in cancer is paradoxical. Initially, it acts as a tumor suppressor by enhancing antioxidant pathways, thus protecting cells from the DNA damage that can lead to cancer. However, once cancer develops, NRF2 can support tumor growth and resistance to chemotherapy by providing cancer cells with enhanced protection against oxidative stress.
Research shows that in the context of cancer, NRF2 activity is often dysregulated. High NRF2 levels in tumor cells lead to an abnormal increase in antioxidant production, promoting cancer cell survival under oxidative stress conditions. This dysregulation is partly due to mutations in KEAP1, a protein that normally helps degrade NRF2, leading to its accumulation and continuous activation.
Furthermore, studies have demonstrated that NRF2 not only increases antioxidant response but also alters metabolic pathways and drug efflux mechanisms in cancer cells, contributing to chemotherapy resistance. This dual role of NRF2, as both protector against and promoter of cancer, underlines its complexity in cancer biology and highlights the challenges in targeting NRF2 pathways therapeutically.
Therapeutic Potential of NRF2
How does targeting NRF2 offer potential therapeutic avenues in the treatment of cancer, given its dual role in tumor development and progression?
Research indicates that selectively modulating NRF2 activity can either inhibit cancer cell growth or protect normal cells from oxidative stress-induced damage, depending on the context. For instance, inhibiting NRF2 in cancer cells that overexpress this factor can decrease their survival and proliferation by increasing oxidative stress and reducing detoxification capabilities.
This strategy leverages the fact that some cancer cells are 'addicted' to NRF2's protective effects against the oxidative stress they generate.
Conversely, activating NRF2 in normal cells or in early stages of cancer development can enhance their resistance to oxidative damage, potentially preventing the initiation and progression of cancer. This approach exploits NRF2's ability to upregulate antioxidant and detoxification genes, thereby safeguarding cells from mutations and malignancy.
Evidence supports the use of specific NRF2 activators or inhibitors, tailored to the cancer type and stage, as a promising strategy for cancer therapy. However, the therapeutic efficacy of NRF2 modulation relies on a precise understanding of its role in the specific cancer context, necessitating further research to optimize these interventions.
Challenges in NRF2 Targeting
Despite the promising therapeutic potential of NRF2 modulation in cancer treatment, several challenges hinder its clinical application, primarily due to the complex biology of NRF2 signaling. You must understand that NRF2's role isn't one-dimensional; it acts as a double-edged sword. While NRF2 activation offers cytoprotective effects against oxidative stress, its overactivation can promote cancer cell survival and resistance to chemotherapy. This duality complicates the development of NRF2-targeted therapies, as you need strategies that can discern between its protective and oncogenic roles.
Moreover, the specificity of NRF2 modulators poses another significant challenge. Current modulators lack the precision to exclusively target NRF2 without affecting other signaling pathways, leading to potential off-target effects and toxicity. This specificity issue underscores the need for improved drug design and screening methods to identify more selective NRF2 modulators.
Lastly, the genetic and epigenetic variations in NRF2 among different cancers and even within the same tumor type add another layer of complexity. You're dealing with a moving target, as these variations can influence the efficacy of NRF2-targeted therapies. This calls for personalized medicine approaches, requiring robust biomarkers to predict response to NRF2 modulation and guide treatment decisions effectively.
Frequently Asked Questions
How Does Dietary Intake Influence NRF2 Activation and Oxidative Stress in the Context of Cancer Prevention?
Your dietary intake plays a crucial role in activating NRF2 and managing oxidative stress, which is key in cancer prevention. Antioxidant-rich foods, like fruits and vegetables, boost NRF2 activation, enhancing your body's defense against oxidative damage.
This activation supports the detoxification of carcinogens and the reduction of oxidative stress, ultimately lowering cancer risk. By focusing on a diet rich in antioxidants, you're directly influencing NRF2's protective mechanisms against cancer development.
Can Lifestyle Factors Such as Exercise and Stress Management Alter NRF2 Activity and Its Impact on Cancer Progression?
Yes, your lifestyle choices, like regular exercise and effective stress management, can indeed influence NRF2 activity and its role in cancer progression.
Engaging in physical activity boosts NRF2 signaling, enhancing your body's antioxidant defenses. Similarly, managing stress effectively reduces oxidative stress, indirectly supporting NRF2's protective functions.
Together, these actions can mitigate cancer risk by modulating the NRF2 pathway, underscoring the importance of a healthy lifestyle in cancer prevention.
Are There Any Genetic Predispositions That Affect an Individual's NRF2 Response to Oxidative Stress, Potentially Influencing Cancer Susceptibility?
Yes, you're likely predisposed to certain NRF2 responses due to your genetics, which can alter how you handle oxidative stress and influence your cancer risk.
Studies show variations in the NRF2 gene affect its activity and the body's defense against oxidative damage.
This means you're not just at the mercy of lifestyle choices; your genetic makeup plays a crucial role in your susceptibility to cancer through its interaction with oxidative stress mechanisms.
How Does the Interaction Between NRF2 and Other Signaling Pathways, Such as Inflammation or Autophagy, Contribute to Cancer Development or Treatment Resistance?
You're exploring how NRF2's interaction with signaling pathways like inflammation or autophagy impacts cancer progression or treatment resistance.
It's crucial to understand that NRF2, while defending cells against oxidative stress, can also cross-talk with these pathways, potentially contributing to cancer's complexity.
This interaction might influence how cancer cells grow, evade death, or resist treatments, highlighting the importance of targeting these pathways alongside NRF2 in developing effective cancer therapies.
What Role Does the Microbiome Play in Modulating NRF2 Activity and Its Effects on Oxidative Stress and Cancer?
You're exploring how the microbiome influences NRF2 activity, impacting oxidative stress and cancer development. Research shows the gut microbiota can modulate NRF2 pathways, affecting both stress responses and carcinogenesis.
Specific microbial compositions or metabolites might enhance or suppress NRF2 activity, thereby altering cancer risk or progression. Understanding this relationship offers potential for targeted interventions, aiming to manipulate the microbiome to modulate NRF2 activity and improve cancer outcomes.
Conclusion
In conclusion, you've seen how NRF2 plays a dual role in cancer, acting both as a protector by mitigating oxidative stress and as a potential promoter of tumor growth.
The therapeutic targeting of NRF2 holds promise, yet it's fraught with complexities due to its dual nature.
Navigating these challenges requires a nuanced understanding of NRF2's mechanisms and the oxidative stress landscape.
Thus, advancing NRF2-targeted therapies necessitates a balanced approach, leveraging its protective benefits while circumventing its cancer-promoting potentials.
Please validate any information here with a healthcare professional. The content is provided for education purposes, This content has not been evaluated by the Food and Drug Administration. Any advice or products mentioned is/are not intended to diagnose, treat, cure, or prevent any disease,










