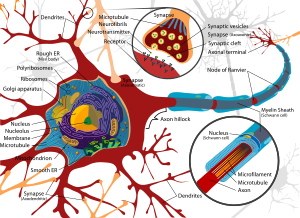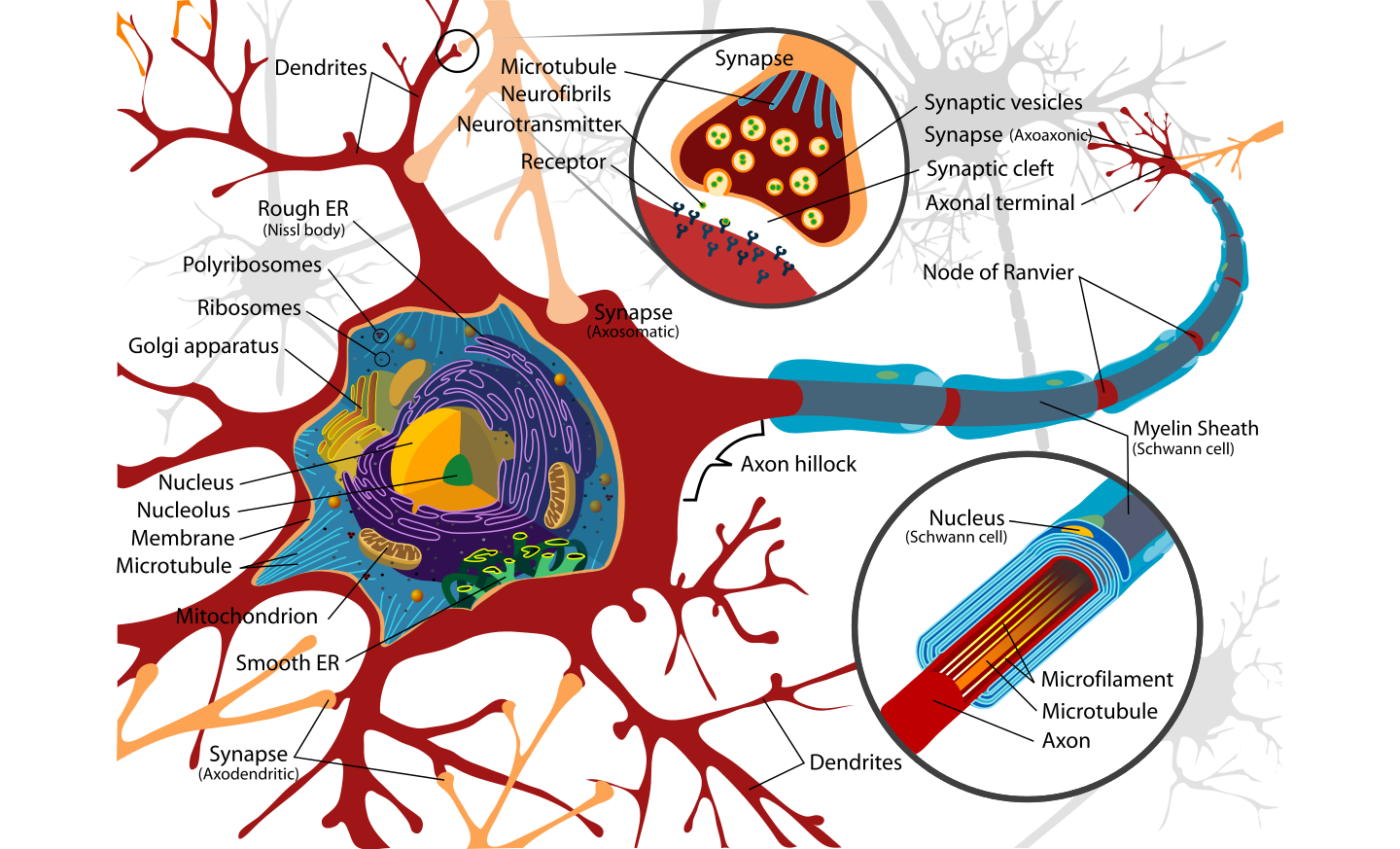
A new study supported by grants from NINDS and the National Institute on Aging as well as funding provided by the Taube/Koret Center, the National Science Foundation , the Huntington’s Disease Society of America, the Milton Wexler Award, and the Hillblom Foundation shows that activating a gene known as NRF2 helps clear damaged proteins which slows down or could possibly prevent Huntington’s disease.
The study broke new ground in developing and using a technique called optical pulse-labeling to measure how quickly damaged proteins get removed. “Before this new technique, there was no way to look at individual neurons and their capacity to handle proteins. This method provides a real-time readout of how fast proteins are turned over in neurons and gives us a look at some of the mechanisms involved,” said Margaret Sutherland, Ph.D., program director at NINDS.
The research studied the impact of different forms of huntingtin, the protein in Huntington’s disease on neuron death and the symptoms of the disease. The experiments showed that the mutant form of huntingtin caused more rat cells to die than the normal healthy form of the protein.
To test this idea, the researchers activated Nrf2, a protein known to regulate protein processing. When Nrf2 was turned on, the mean lifetime of huntingtin was shortened, and the neuron lived longer. The researchers discovered that neuronal survival is directly correlated with the amount of time a neuron is exposed to the mutant huntingtin protein. Improving proteostasis in Huntington’s brains may improve neuronal survival and slow down or even prevent the the progression of the disease.
“Nrf2 seems like a potentially exciting therapeutic target. It is profoundly neuroprotective in our Huntington’s model and it accelerates the clearance of mutant huntingtin,” said Dr. Steven Finkbeiner, senior author of the paper.
“These findings provide evidence that our brains have powerful coping mechanisms to deal with disease-causing proteins. The fact that some of these diseases don’t cause symptoms we can detect until the fourth or fifth decade of life, even when the gene has been present since birth, suggests that those mechanisms are pretty good,” said Dr. Finkbeiner.
Other NRF2 Huntington studies on Pubmed.org:
Please validate any information here with a healthcare professional. The content is provided for education purposes, This content has not been evaluated by the Food and Drug Administration. Any advice or products mentioned is/are not intended to diagnose, treat, cure, or prevent any disease,